Kickmaker is developing a program dedicated to medical fields, especially medical devices & personnal care devices, (any in/on vivo devices), in vitro diagnosis devices, instrumentation & optics (any in vitro devices).

Industrializing medical devices (MDs) is a complex process, which enables us to go from the development phase to mass production, guaranteeing consistent product quality, as well as an appropriate response to strict quality and regulatory requirements.
Adding agility to this process makes it easier to navigate in an environment of fast-changing technologies, standards and regulations, and to meet customers’ growing demand for customization and modularity.
But how can the agile approach and its key principles, well known in the software world, be applied to the development of a product with a hardware component? And what are the associated challenges?
Summary:
-
-
Introducing agility into DM development
-
Same old story: focus on short, iterative development cycles
-
Development by independent modules
-
Working with an external service provider: a hybrid organizational model
-
Conclusion
-
Introducing agility into DM development
Agility refers to the teams’ ability to adapt quickly and efficiently to changing needs, technology and customer requirements. Well-anchored in the world of software (SW), it nevertheless encounters major challenges due to the specificities of hardware compared to software, such as the fixed nature of hardware specifications as opposed to their evolutionary nature in software.
Document traceability, essential in this context, is more complex due to more rigorous review flows. In addition, the verification and validation (V&V) cycle associated with hardware-based medical devices tends to be significantly longer than in software development. Despite these inherent challenges, solutions are emerging to integrate agility into the industrialization process for these medical devices.
Same old story: focus on short, iterative development cycles
Iterative development allows teams to split the process into smaller cycles, regularly reassessing and adjusting the project in line with changing needs and feedback. It allows unnecessary specs to be eliminated, and strict simulated user, verification and performance tests to be carried out throughout the process.
The challenges of traceability and flexibility can be solved with a clean project management method. V-cycle stages (spec, design, design justification, test plans, test reports), risk and impact management (project, product, regulatory), change management (ECR/ECO/ECN) are interconnected, and versioning takes place at the overall level to provide a centralized, dynamic view of project progress. Specifications are used and regularly updated. The risk of errors at an advanced stage is reduced, and early detection of potential problems is enabled, which will limit the financial impact of the design changes needed to correct them.
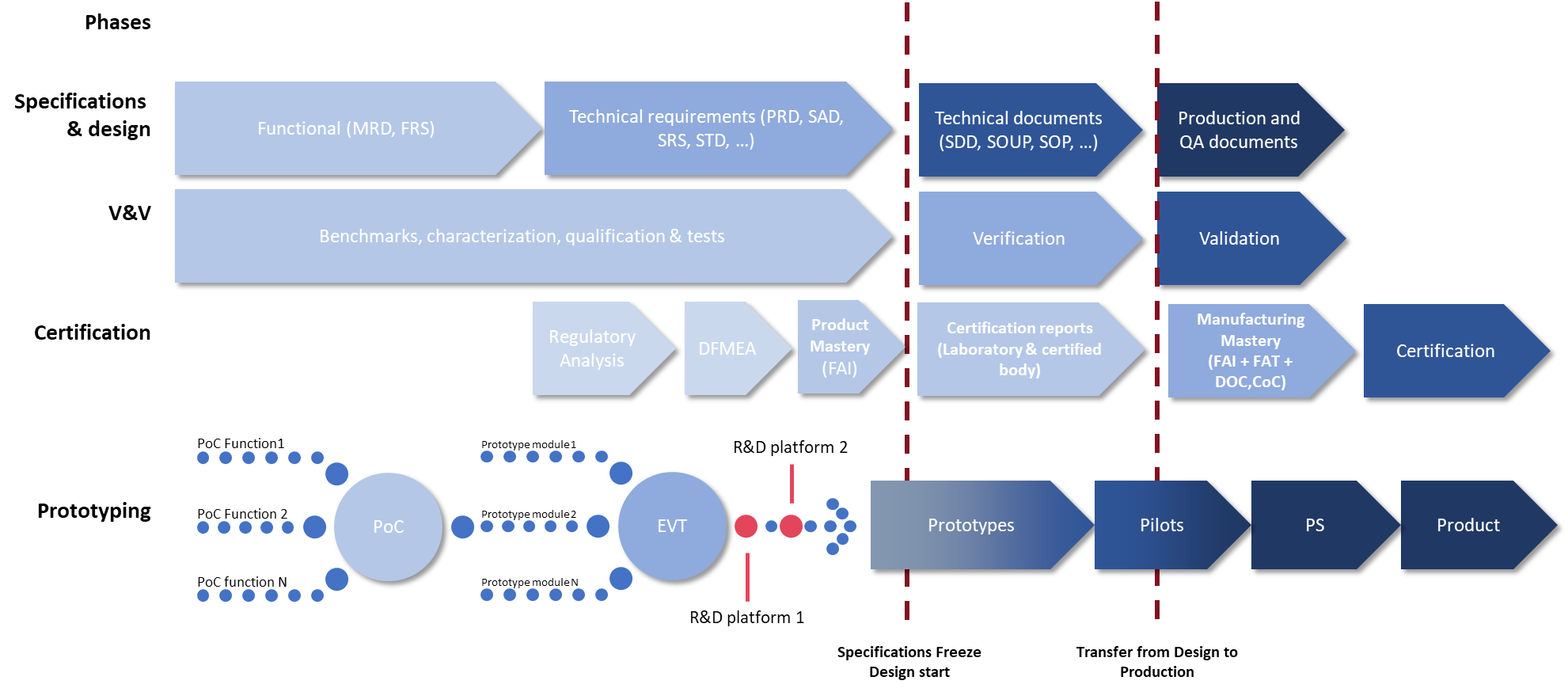
Development by independent modules
Flexibility is essential to ensure room for change. By designing the DM in independent bricks from the outset, we add a considerable degree of scalability. Functionalities are separated, particularly medical and non-medical ones, which means that the entire product is not re-documented each time it is modified, but only the impacted module; and functionalities can be added during the industrialization process.

Working with an external service provider: a hybrid organizational model
A third model may find its salvation in a hybrid form: a competence center. This concerns well-defined scopes of activity, which require greater flexibility. At the start of the project, a complete backlog of tasks to be carried out is drawn up with the customer, to which a specific team is attached. Each member of the team is assigned an occupation rate, and every month a sprint is run on the defined activities. At the end of the month, the completed tasks are listed and the backlog updated: should we staff more? Or less? Have the specifications changed? Tasks are defined and updated month by month. This method adds a great deal of agility to the design process.
Conclusion
Balancing agility with the rigorous processes required for medical device development is a delicate but necessary aspect of successful implementation. While hardware agility will never be as flexible as sotfware agility, it does enable the creation of innovative, user-centric solutions.



Recent Comments